Confused about which COVID-19 test is right for you?
To face the current COVID-19 pandemic, diagnostic tools are essential. It is recommended to use real-time RT-PCR for RNA viruses in order (a) to perform a rapid and accurate diagnostic, (b) to guide patient care and management, and (c) to guide epidemiological strategies.
Real-time RT-PCR remains the reference method for the diagnosis of SARS-CoV-2 infection. On the other hand, notwithstanding its varying sensitivity according to the time of infection, serology represents a valid asset (a) to try to solve possible discrepancies between a highly suggestive clinical and radiological presentation and negative RT-PCR, (b) to solve discrepancies between different PCR assays, and (c) for epidemiological purposes.
Molecular and serological tests were previously compared during the SARS-CoV-1 epidemic, showing an increased sensitivity and specificity for the molecular ones. For this reason, real-time reverse transcription polymerase chain reaction (RT-PCR) represents the validated assay for early diagnosis in patients with suspected SARS-CoV-2 infection.
Types of popular COVID-19 tests for virus detection:
Rapid Antigen Test
This test detects specific viral protein antigens present on the virus surface from the sample taken from the nose. Although the test results are quick (within 15 to 30 minutes ), it has low sensitivity and produces false-negative results most of the time, and is not widely accepted for clinical diagnosis of COVID-19 infection.
RT-PCR: direct test, real-time reverse transcription polymerase chain reaction
For detecting general materials from SARS-Cov2. COVID-19 only contains RNA (Ribonucleic Acid) materials. This is the gold standard for accurate laboratory methods in the detection of the COVID-19 virus. The RT-PCR test is highly specific; a positive test result is highly confirmatory as dual tests are done before reporting, first for screening and secondarily for confirmation.
Antibody Test: indirect test, for checking immunity.
An antibody test is conducted on a person’s blood sample to detect the presence of antibodies developed in the body against the COVID-19 virus. The positive test result indicates exposure of the person to the virus and is an indicator of developed immunity in the body against the virus. However, there are limitations to this test, and it is not used for the diagnosis of COVID-19 infection. Experts say that antibody tests can be misleading due to false-negative results if done in the first week of the coronavirus exposure.
There are two types of antibodies available: IgM and IgG.
IgM is short-lived and will disappear within days after the virus is no longer active. IgG is produced after the active infection and is present for weeks after the virus is no longer active. The level of these two antibodies gives us an indication of the infection life cycle phase at the time of test (the infection phase and the recovery or post-recovery phase). However, there are several antibody tests available on the market, and the mechanisms of detection are different. For example, some tests only detect the presence of IgM, some only detect IgG, and others detect the presence of both antibodies.
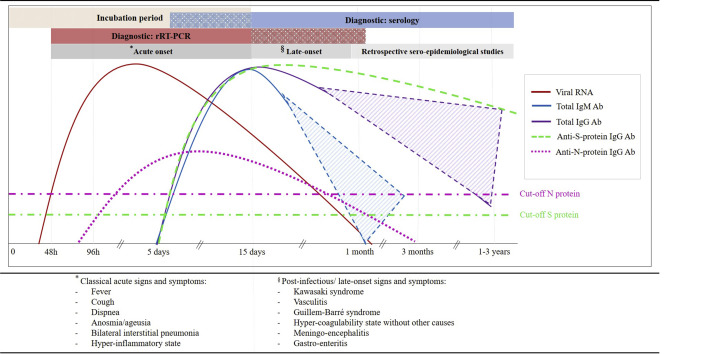
Fig. 1: Kinetics of SARS-CoV-2 markers during infection and laboratory diagnosis. RT-PCR, real-time reverse transcription polymerase chain reaction; RNA, ribonucleic acid; IgM, immunoglobulin type M; IgG, immunoglobulin type G; Ab, antibodies (2).
- WHO.int, Laboratory testing for coronavirus disease 2019 (COVID-19) in suspected human cases: interim guidance. World Health Organization, 2020 https://apps.who.int/iris/handle/10665/331329
- G. Caruana, A. Croxatto, A.T. Coste, O. Opota, F. Lamoth, K. Jaton, G. Greub . Diagnostic strategies for SARS-CoV-2 infection and interpretation of microbiological results Clinical Microbiology and Infection Volume 26 Issue 9 Pages 1178-1182 (September 2020)DOI: 10.1016/j.cmi.2020.06.019)